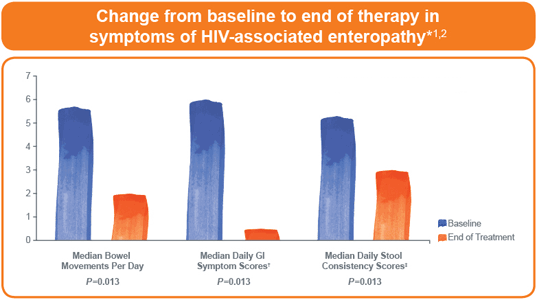
- Marked improvements in GI-related symptoms were seen in all patients within
3 weeks - The patients’ CD3+/CD4+ T-cell density increased from 213 to 322 cells/mm2–a median increase of 140 cells/mm2 (P=0.016)
*The clinical trial was an 8-week, open-label study of EnteraGam™ administered at a dosage of 2.5 g twice daily to 8 patients who had severe HIV-associated enteropathy with approximately 5 to 6 watery stools per day.1,2
†GI symptom questionnaire assessed cramping, urgency, incontinence, and nocturnal diarrhea, with a possible score of 0 to 24, with <2 being normal.1,2
‡Scale of 1 (formed) to 6 (watery).1,2
Please see full Important Safety Information in Safety section and
full Prescribing Information including contraindications in PI section.
full Prescribing Information including contraindications in PI section.